G-protein metallochaperones ensure fidelity during cofactor insertion to target metalloproteins, including adenosylcobalamin (AdoCbl)-dependent methylmalonyl-CoA mutase, hydrogenases and ureases. In general, our understanding of cofactor biogenesis and cofactor delivery is modest compared to what we know about the enzymology of metalloproteins. In many cases, this lack of information hinders biotech applications, as overproduction of target metalloenzymes is not possible without complete knowledge of the cofactor assembly/delivery process. Additionally, impairment in delivery and assembly in humans is associated with diseased states. The Drennan lab probes the molecular mechanisms of cofactor assembly and biogenesis (also see Section II on Radical SAM enzymes that are involved in cofactor biosynthesis and Section V on iron-sulfur cluster biogenesis).
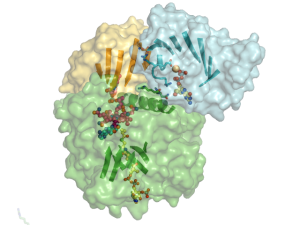
In our work on cobalamin-dependent enzyme maturation, we solved the first structure of a metallochaperone domain (blue in accompanying figure) with its target mutase (cobalamin domain in yellow, catalytic domain in green) and have discovered that the cobalamin domain and chaperone domain form a continuous β-sheet that allows the domains to move in unison, thus opening and closing the enzyme for cobalamin assembly. We are currently investigating the molecular basis for enhanced GTPase activity when a metallochaperone binds its target enzyme in our on-going studies in this area.
Publications(*Corresponding authors)
Vaccaro, F.A., Faber, D.A., Andree, G.A., Born, D.A., Kang, G., Fonseca, D.R., Jost, M., and Drennan*, C.L. (2023) Structural insight into G-protein chaperone-mediated maturation of a bacterial adenosylcobalamin-dependent mutase, J. Biol. Chem. 299, 105109. doi: https://doi.org/10.1016/j.jbc.2023.105109 PMCID: PMC10481361. PMID: 37517695
Vaccaro, F.A., Born, D.A, and Drennan*, C.L. (2023) Structure of metallochaperone in complex with the cobalamin-binding domain of its target mutase provides insight into cofactor delivery, Proc. Natl. Acad. Sci. doi: 10.1073/pnas.2214085120. PMCID: PMC9974510. PMID: 36787360
Jost, M., Cracan, V., Hubbard, P.A., Banerjee, R., and Drennan*, C.L. (2015) Visualization of a Radical B12 Enzyme with its G-protein Chaperone, Proc. Natl. Acad. Sci. U.S.A. 112, 2419-2424. PMCID: PMC4345561
Hubbard, P.A., Padovani, D., Labunska, T., Mahlstedt, S.A., Banerjee, R., and Drennan*, C.L., (2007) Crystal Structure and Mutagenesis of the Metallochaperone MeaB: Insight into the Causes of Methylmalonic Aciduria, J. Biol. Chem. 282, 31308-31316. DOI: 10.1074/jbc.M704850200
Review
Vaccaro, F.A., Born, D.A, and Drennan*, C.L. (2023) Structure of metallochaperone in complex with the cobalamin-binding domain of its target mutase provides insight into cofactor delivery, Proc. Natl. Acad. Sci. doi: 10.1073/pnas.2214085120. PMCID: PMC9974510. PMID: 36787360