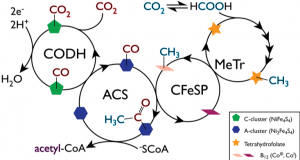
The Wood-Ljundahl (W-L) pathway of carbon dioxide fixation takes two molecules of carbon dioxide and reduces them to form the acetate moiety of acetyl-CoA. Reducing equivalents for this pathway can be provided by hydrogen gas or from the oxidation of 2-oxoacids such as oxalate via 2-oxoacid:ferredoxin oxidoreductases. The W-L pathway, which is thought to be ancient, is unique in that it attaches two carbon units directly to each other rather than adding carbon dioxide to an already formed carbon chain. It requires multiple oxygen-sensitive metalloproteins and two unique Ni-Fe-S clusters referred to as the C-cluster and A-cluster. Both C- and A-clusters play a major role in the global carbon cycle and in the formation and removal of greenhouse gases and CO in our environment. An estimated 108 tons of CO are removed from the lower atmosphere and earth annually by C-cluster-containing bacteria, and an estimated 1010 tons of acetate is produced from the process of acetogenesis.
To make acetyl-CoA from carbon dioxide, the C-cluster of the enzyme carbon monoxide dehydrogenases (CODH) converts carbon dioxide to carbon monoxide, the biological equivalent of the water-gas shift reaction. The A-cluster of acetyl-CoA synthase (ACS) takes the CO generated by the C-cluster and combines it with a methyl group and CoA to make acetyl-CoA. The methyl group used to make acetyl-CoA, also derived from carbon dioxide, is delivered to ACS by the corrinoid-Fe-S protein (CFeSP).
Together with Steve Ragsdale (U Michigan), the Drennan lab has determined structures of the complex between CODH and ACS and of the complex between CFeSP and the methyl transferase MeTr, all from the model acetogen Moorella thermoacetica. The Drennan lab has also solved structures of the two 2-oxoacid:ferredoxin oxidoreductases from Moorella, which are known to provide reducing equivalents. But there is much more to do. We are missing structural information about the methyl transfer from CFeSP to ACS, a unique metal to metal transfer of a methyl moiety. We are also missing structural information about ligand-bound states of the A-cluster and mechanistic details about the A-cluster reaction, which is the biological equivalent of the Monsanto process. We are currently using both crystallography and EM to pursue these structural data.
Press
Highlighted in https://chemistry.mit.edu/chemistry-news/new-snapshots-of-ancient-life/
Faculty of 1000 recommendation, Aug 30, 2019: Wittenborn et al. “Structural Insight into Metallocofactor Maturation in Carbon Monoxide Dehydrogenase”
MIT News Dec 28, 2015, “A Healthy Breakdown: Researchers Discover How Some Organisms Process Oxalate, a Molecule that Can Harm Humans” by Peter Dizikes
C&EN: “Methyl Handoff Between B Vitamins” by Amanda Yarnell, March 19, 2012.
University of Michigan Medicine March 12, 2012, “Vitamins Doing Gymnastics: Scientists Capture First Full Image of Vitamin B12 in Action”
Publications(‡Co-first authors, *Corresponding authors)
Biester, A., Grahame, D.A., Drennan*, C.L. (2024) Capturing a Methanogenic Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase Complex via Cryogenic Electron Microscopy Proc. Natl. Acad. Sci. 121 (41) e2410995121 https://doi.org/10.1073/pnas.2410995121 PMCID: PMC11474084. PMID: 39361653
Biester, A., Dementin, S., and Drennan*, C.L. (2022) Visualizing the Gas Channels of a Monofunctional Carbon Monoxide Dehydrogenase, J. Inorg. Biochem. 230, 111774. Special issue in honor of Dick Holm. doi: 10.1016/j.jinorgbio.2022.111774. PMCID: PMC9093221. PMID: 35278753
Cohen, S.E., Brignole, E.J., Wittenborn, E.C., Can, M., Thompson, S., Ragsdale, S.W., and Drennan,* C.L. (2020) Negative-Stain Electron Microscopy Reveals Dramatic Structural Rearrangements in Ni-Fe-S-Dependent Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase, Structure 29: 43-49.E3.
Cohen, S.E., Can, M., Wittenborn, E.C., Hendrickson, R.A., Ragsdale, S.W., and Drennan*, C.L. (2020). Crystallographic Characterization of the Carbonylated A-Cluster in Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase, ACS Catal. 10:9741-9746.
Wittenborn, E.C., Cohen, S.E., Merrouch, M., Léger, C., Fourmond, V., Dementin*, S., and Drennan*, C.L. (2019) Structural Insight into Metallocofactor Maturation in Carbon Monoxide Dehydrogenase, J. Biol. Chem. 294, 13017-13026. PMCID: PMC6721931.
Chen‡, P.Y-T., Li‡, Bin, Drennan*, C.L., and Elliott*, S.J. (2019) A Reverse TCA Cycle 2-Oxoacid:Ferredoxin Oxidoreductase that Makes C-C Bonds from CO₂, Joule 3, 595-611. PMCID: PMC6508887
Chen, P.Y.-T., Aman, H., Can, M., Ragsdale, S.W., and Drennan*, C.L. (2018) Binding Site for Coenzyme A Revealed in the Structure of Pyruvate:Ferredoxin Oxidoreducatase from Moorella thermoacetica Proc. Natl. Acad. Sci. U.S.A. 115, 3846-3851. PMCID: PMC5899475 [Available on 2018-10-10]
Gibson, M.I., Chen, P. Y.-T., Johnson, A.C., Pierce, E. Can, M., Ragsdale, S.W., Drennan*, C.L. (2016) One-Carbon Chemistry of Oxalate Oxidoreductase Captured by X-Ray Crystallography, Proc. Natl. Acad. Sci. U.S.A., 113, 320-325. PMCID: PMC4720323
Gibson, M.I., Brignole, E.J., Pierce, E., Can, M., Ragsdale, S.W., and Drennan*, C.L. (2015) The Structure of an Oxalate Oxidoreductase Provides Insight into Microbial 2-Oxoacid Metabolism, Biochemistry 54, 4112-4120. PMCID: PMC4498597
Ando, N., Kung, Y., Can, M., Bender, G., Ragsdale, S.W., and Drennan*, C.L. (2012) Transient B12-Dependent Methyltransferase Complexes Revealed by Small-Angle X-ray Scattering, J. Am. Chem. Soc. 134, 17945-17954. PMCID: PMC3484714
Kung, Y., Ando, N., Doukov, T.I., Blasiak, L.C., Bender, G., Seravalli, J., Ragsdale, S.W., and Drennan*, C.L. (2012) Visualizing Molecular Juggling within a B12-dependent Methyltransferase Complex, Nature 484, 265-269. PMCID: PMC332619 Faculty of 1000 Recommended
Kung, Y., Doukov, T.I., Seravalli, J., Ragsdale, S.W., and Drennan*, C.L. (2009) Crystallographic Snapshots of Cyanide- and Water-Bound C-Clusters from Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase, Biochemistry 48, 7432-7440. PMCID: PMC2721637
Doukov, T.I., Blasiak, L.C., Seravalli, J., Ragsdale, S.W., and Drennan*, C.L. (2008) Xenon in and at the End of the Tunnel of Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase, Biochemistry 47, 3474-3483. PMCID: PMC3040099
Doukov‡, T.I, Hemmi‡, H., Drennan, C.L., and Ragsdale*, S.W. (2007) Structural and Kinetic Evidence for an Extended Hydrogen-Bonding Network in Catalysis of Methyl Group Transfer. Role of an Active Site Asparagine Residue in Activation of Methyl Transfer by Methyltransferases, J. Biol. Chem. 282, 6609-6618. PMCID: PMC3966722 Faculty of 1000 Recommended
Doukov, T.I., Iverson, T.M., Seravalli, J., Ragsdale, S.W., and Drennan*, C.L. (2002) A Ni-Fe-Cu Center in a Bifunctional Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase, Science 298, 567–572. Faculty of 1000 Recommended
Drennan*, C.L., Heo, J., Sintchak, M.D., Schreiter, E., and Ludden, P.W. (2001) Life on Carbon Monoxide: X-ray Structure of Rhodospirillum rubrum Ni-Fe-S Carbon Monoxide Dehydrogenase, Proc. Natl. Acad. Sci. U.S.A. 98, 11973–11978. PMCID: PMC59822 Faculty of 1000 Recommended
Review Article/Book Chapters
Biester, A., Marcano-Delgado, A.N., Drennan*, C.L. (2022) Structural insights into microbial one-carbon metabolic enzymes Ni−Fe−S-dependent carbon monoxide dehydrogenases and acetyl-CoA synthases, Biochemistry 61(24), 2797-2805. doi: 10.1021/acs.biochem.2c00425. PMCID: PMC9782325. PMID: 36137563
Andorfer*, C.A., and Drennan, C.L. (2021) Fixing Nature’s Carbon Inefficiencies, Joule 5, 765-767. PMC exempt, review article; DOI: 10.1016/j.joule.2021.03.025
Kung*, Y., and Drennan, C.L. (2017) One-Carbon Chemistry of Nickel-Containing Carbon Monoxide Dehydrogenase and Acetyl-CoA Synthase in The Biological Chemistry of Nickel, Deborah Zamble, Magdalena Rowińska-Żyrek and Henryk Kozlowski (eds), Cambridge, UK: Royal Society of Chemistry, pp. 121–148. PMC exempt, book chapter; DOI: 10.1039/9781788010580-00121
Gibson, M.I., Chen, P.Y.-T., and Drennan*, C.L. (2016) A Structural Phylogeny for Understanding 2-Oxoacid Oxidoreductase Function, Curr. Opin. Struct. Biol. 41, 54-61. PMCID: PMC5381805
Kung, Y., and Drennan*, C.L. (2011) A Role for Nickel-iron Cofactors in Biological Carbon Monoxide and Carbon Dioxide Utilization, Curr. Opin. Chem. Biol. 15, 276-283. PMCID: PMC3061974