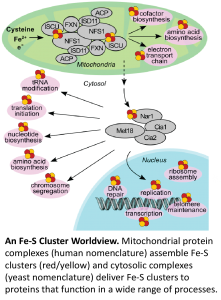
Iron-sulfur cluster biogenesis is a process that in humans is associated with upwards of twelve different medical conditions, including Friedreich’s ataxia, and several forms of mitochondrial dysfunction. Iron-sulfur clusters are functionally versatile cofactors found in all kingdoms of life, and although they form spontaneously in vitro, the process in vivo is complex, highly regulated and enigmatic.
The Drennan lab is collaborating with the Barondeau lab (Texas A&M) on studies of the mitochondrial Fe-S cluster biogenesis machinery and with the Perlstein lab (Boston U) on studies of the cytosolic protein machinery, which is responsible for delivering Fe-S clusters. We are excited to employ anaerobic cryo-EM to investigate the protein complexes involved in iron-sulfur cluster biogenesis and delivery. We have established the laboratory for anaerobic cryo-EM (ACE@MIT) for these (and other) investigations of oxygen-sensitive metalloprotein complexes.
Press
2023 Highlighted by MIT News: https://biology.mit.edu/news/news-briefs-drennan-lab/
SSRL Science Highlight September 30, 2017, “Structure of the Human Cysteine Desulfurase Complex”.
Publications(*Corresponding author)
Vasquez, S., Marquez, M.D., Brignole, E.J., Vo, A., Kong, S. Park, C., Perlstein*, D.L., and Drennan*, C.L. (2023) Structural and biochemical investigations of a HEAT-repeat protein involved in the cytosolic iron-sulfur cluster assembly pathway, Commun. Biol. 6, 1276. doi:10.1038/s42003-023-05579-3. PMID: 38110506
Cory, S.A., Van Vranken, J.G., Brignole, E.J., Patra, S., Drennan, C.L., Rutter, J., and Barondeau*, D.P. (2017) Structure of Human Fe-S Assembly Subcomplex Reveals Unexpected Cysteine Desulfurase Architecture and Acyl-ACP-ISD11 Interactions, Proc. Natl. Acad. Sci. U.S.A. 114, E5325-E5334. PMCID: PMC5502623 F1000 recommended.